
Recognizing: the decision of the 57th Ordinary Session of the ECOWAS
Authority of Heads of States and Governments held on the 7th
of September 2020 in Niamey, Niger to constitute Regional
Taskforce to conduct a feasibility study on the production of
vaccines.
Recognizing: the decision of the 58th Ordinary Session of the ECOWAS
Authorities of Heads of States and Governments held virtually on
21 January 2021 to develop a strategy for the availability of anti-
COVID-19 vaccines in the ECOWAS region and establish the
Regional Revolving Fund for the pooled procurement mechanism
to accelerate access of vaccines.
Noting: the Africa CDC and PAVM-Framework for Action meeting held in Kigali,
Rwanda from 06 to 07 December 2021, on the fourth objective to build the
momentum of vaccine manufacture hubs and pilot a drug API-final drug
product Hub program.
Noting: that the ECOWAS region’s share 35% of vaccines market volume demand
on the Africa continent, driven by population size.
We the stakeholders in Vaccines Manufacturing in the West Africa Region under the leadership of ECOWAS/WAHO and Africa CDC met in Accra, Ghana from May 10th to 11th, 2022 to discuss on how to collaborate to ensure the production and availability of quality, safe and efficacious vaccines in the region.
The main objective of the high-level meeting is to create a framework for collaboration, information exchange and develop a framework for vaccines producers in the West African region to discuss strategically how to support each other build the regional hub for vaccines production.
Specifically, the meeting aims to:
- Identify the bottlenecks in vaccines research and development and production.
- Identify and define the types of vaccines to be produced for the region by each manufacturer.
- Define short, medium, and long-term strategies to boost vaccines production in the region
- Engage more partners and biotechnology developers to liaise with manufacturers.
Background
- The ability to manufacture hundreds of millions to billions of doses of vaccines requires the vaccine-manufacturing capacity of the entire world. (Corey et al 2020., Science, 368: 948-950).
- Africa has around 16% of the world’s population and carries about 25% of the world’s global disease burden. Over 50% of the world’s infectious diseases burden (HIV, Ebola, Lassa fever, etc…) is borne by Africa. Less than 1% of the vaccines required by Africans are produced in Africa, creating a significant health security risk.
- Building regional manufacturing capabilities will contribute to pandemic preparedness and strengthening the response to future outbreaks. This should include training in current Good Manufacturing Practices (cGMP), Regulatory issues, etc. Consideration should also be given to investing in pre-clinical research and development (R&D) activities as well to drive more ownership of the R&D value chain in the region and the continent.
- Investing in late-stage clinical trials, trial sites and production capacity for endemic/pandemic disease products could improve access to vaccines, therapeutics, and diagnostics in Africa.
- Economic returns would be especially high if new vaccines were produced for regional markets rather than narrow domestic markets only. Each country can also seize the African Continental Free Trade Area (AfCFTA) opportunity to trade with other countries outside the West African region. This means that developing and producing vaccines for a larger group of countries is favorable compared to narrowly targeting domestic markets, where currently 99% of Africa’s vaccines are imported due to very limited vaccine upstream and downstream production.
Recommendations:
- The meeting agreed on types of vaccines to be produced in the region into considering that diversification of vaccines products (combining child immunization programs and adult vaccination programs that will require demand generation) will also help support manufacturers.
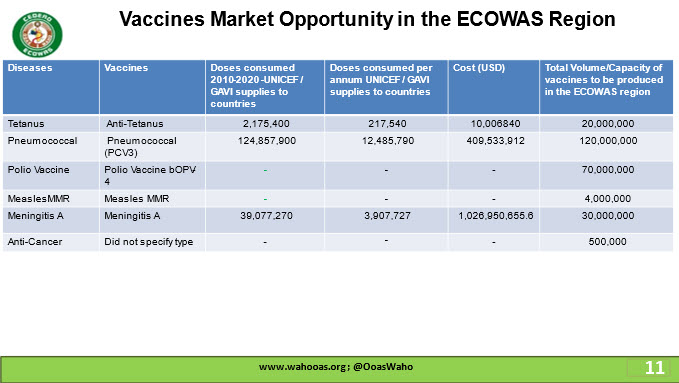
This includes twenty-two (22) identified vaccines which were further divided into 3 based on current and planned vaccine manufacturer’s capacities and capabilities in s terms of short-term, medium-term and long-term depending on the period of production.
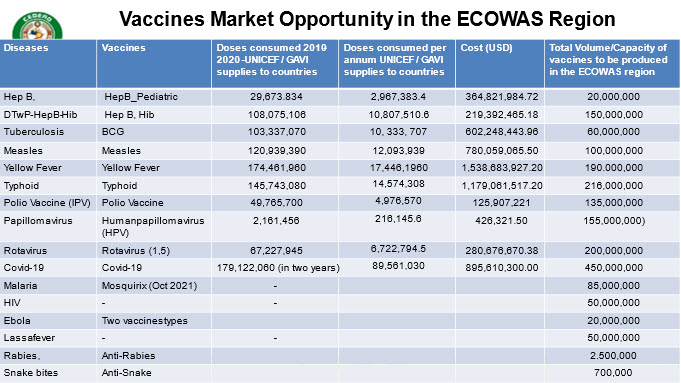
Short term: Yellow Fever vaccines, Anti snake serum, Covid-19 vaccines, Pentavalent, Rabies, Measles, Ebola, Typhoid, Lassa, Rotavirus, Tetanus and Polio Vaccines (IPV)- 2022 to 2024
Medium term: Human papillomavirus (HPV), Pneumococcal (PCV3), Polio Vaccine bOPV-4 (oral), Malaria, Measles MMR, BCG, HepB-Pediatric, Meningitis A and 2025 -2027
Long-term: Anti-cancer, 2028 to 2030
- There is a need for collaboration among manufacturers to prevent duplication of efforts. Focus should be on building sustainable bio-manufacturing capacity to meet regional health needs. Such an aspiration will require a completely new way of thinking than what is currently being planned. It should be borne in mind that multiple Fill and Finish plans for COVID-19 vaccines outstrip the needs or current demands). For example, the current mRNA technology will need to be utilized to develop other priority diseases. The region can work closely with Partnerships for African Vaccines Manufacturing (PAVM) which will have oversight of vaccines manufacturing activities in the region and the continent.
- ECOWAS countries and partners should deliberately support manufacturers to de-risk their investments, finance/funds e.g. Advance purchase commitments, grants, soft loans, and other regional or country specific incentives. Strengthening routine immunization programmes will also demand for locally manufactured vaccines.
- ECOWAS/WAHO should play the role to build human resource capital/training, support research development, infrastructure /equipment, logistics and market shaping (design) and technology transfer.
- R&D capacity available in the region needs to be strengthened and utilized in complementary fashion to fulfil gaps in the vaccine R&D value chain on the African continent. Further, the great potential regional organizations offer in manufacturing and R&D talent development should be fully explored and utilized to support the continental needs.
- ECOWAS/WAHO should establish pool procurement to guarantee offtake of vaccines in the region. (Discussion should start with plans to develop strategic stockpiles and distribution plans to support national vaccination programs. That will mean that countries will have to quantify their national needs, pool it across the regional, and use that to provide advanced market commitment to the industry over extended periods of time – at least 5yrs. This way the industry will be able to manage idle facilities).
- ECOWAS/WAHO should also consider endorsing and engaging in a continental pooled procurement mechanism- the Africa Vaccines Acquisition Task Team (AVATT) and get all institutions such as COVAX facility, GAVI, UNICEF and other partners to purchase vaccines produced in the region for its population. This will help drive the continental ambition of growing vaccines manufacturing across other regions as well.
- ECOWAS governments should work to enhance existing trans-border trade policies to enable sale of vaccines within and across the region.
- There is an urgent need to implement harmonization of NRAs and the establishment of the ECOWAS Regional Medicines Agency (ECOMA) in the region and link to Africa Medicines Regulatory Harmonization (AMRH) and Africa Medicines Agency (AMA) to support implementation of the continental strategy in line with the framework for action of PAVM/Africa CDC, with the support from WHO to coordinate for optimal implementation of activities on the continent.
- Market fragmentation will kill all interventions to get vaccines and biologics to the market. Market approvals must be regional in the minimum or continental. There’s a need that this becomes the immediate area of work for ECOMA and AMRH/AMA.
- Regional or continental approval decisions will have to be domesticated by individual countries.
- The need to strengthen individual NMRAs regulatory systems in the ECOWAS region and improve on current good manufacturing practices (cGMP) to international standards for the sustainability of the vaccines manufacturing.
- The need to strengthen relationship with the United Nations Economic Commission for Africa (ECA) reaffirm its support and commitment to continue working with all relevant partners to transform ideas into action for the Africa.
REQUESTS:
- Heads of Governments, Health Ministers, Ministers of Industries and relevant Ministries to help facilitate adoption of the recommendations of this meeting by providing stronger political commitment.
- ECOWAS/WAHO to engage stakeholders, technical and financial partners to fund the vaccines manufacturing in the region.
- That national vaccines manufacturing plans are aligned with the regional and continental plans to prevent duplication of efforts.
- ECOWAS/WAHO to follow up with stakeholders to ensure that the regional priorities are achieved.
